Executive Summary
Drug discovery is entering a decisive phase of reinvention. What was once a slow, sequential, and capital-intensive pipeline is being reshaped by AI Driven Drug Discovery, cloud-based high-performance computing (HPC), and the early emergence of quantum computing as a future simulation engine.
This is not a marginal efficiency gain. It is a structural shift in how biology is interpreted, how molecules are designed, and how therapeutic hypotheses are validated. AI can learn from decades of experimental evidence and generate high-probability candidates faster. Cloud infrastructure delivers supercomputing capacity on demand, enabling large-scale model training and simulation without fixed on-premise constraints. Quantum computing is beginning to redefine what is computationally feasible, particularly for molecular systems where classical approximations introduce meaningful limits.
Together, these technologies are enabling a faster, more predictive, and more collaborative discovery model, where algorithms, automated laboratories, and cloud platforms operate alongside scientific experts to compress timelines and reduce attrition. NexRevo Bioinformatics supports life science organizations globally by designing AI-ready architectures, deploying scalable cloud infrastructure, and building advanced computational workflows that accelerate R and D from early discovery through clinical transition.
The Technology Inflection Point
Drug discovery has always been shaped by the interplay of biology, chemistry, computation, and clinical insight. Yet even with scientific progress, the end-to-end process remains long and uncertain. Development cycles still span many years, cost is high, and most candidates fail before approval, often after significant time and investment.
The root causes are structural. Chemical space is effectively infinite relative to what can be explored experimentally. Biological pathways are complex, context-sensitive, and frequently non-linear. Wet-lab work is slow and resource-intensive. Predictive confidence is often limited in early stages, which pushes risk into later phases where failure is expensive.
AI Driven Drug Discovery, cloud computing, and quantum innovation directly address these constraints. AI enables hypothesis generation and prioritization at scale. Cloud HPC makes large model training and high-throughput molecular evaluation operationally achievable. Quantum computing introduces a longer-term path toward higher-fidelity simulation and optimization. The shift underway is a move from linear trial-and-error to a continuous, data-driven, and increasingly predictive system.
Why the Discovery Workflow Is Changing
Traditional discovery workflows rely on sequential experimentation, with computational methods often limited to supporting roles. AI Driven Drug Discovery changes this by making computation central to decision-making rather than downstream validation.
Machine learning models can integrate historical assay data, molecular features, clinical outcomes, and literature evidence to guide which targets and compounds should be prioritized. Instead of synthesizing broadly and discovering failure later, teams can predict activity, toxicity, and developability earlier, reducing wasted cycles and improving program focus.
Cloud computing is what enables this at real scale. Training modern deep learning models, screening large chemical libraries, and running high-throughput simulations require elastic access to GPUs, storage, and distributed compute. Cloud HPC provides this elasticity while enabling secure collaboration across distributed teams and partners. For many organizations, cloud-based compute also changes the economics by making advanced infrastructure accessible without long capital investment, allowing smaller teams to operate at enterprise-grade scale.
AI Driven Drug Discovery is also changing how R and D organizations operate at the portfolio level. As discovery becomes more computational and data-centric, leaders can move from intuition-led prioritization to evidence-weighted decision-making, continuously re-ranking targets and programs based on real-time signals from omics data, screening results, and early safety indicators. This improves capital allocation, reduces time spent on low-probability programs, and creates a more adaptive pipeline that can respond quickly to new biology, competitive movement, or emerging clinical insights. In practice, the organizations that win will not simply adopt new tools. They will build an operating model where data, automation, and scientific expertise work as a unified system, turning faster learning into faster breakthroughs.
Where AI Is Creating Real R and D Acceleration
AI Driven Drug Discovery is most valuable where it changes decisions early and reduces downstream risk. Its core impact is not only speed, but improved prioritization and a tighter loop between prediction and validation.
Target identification and validation, historically among the slowest steps, can be accelerated through AI systems that analyze genomics, transcriptomics, protein interaction networks, phenotypic screens, and clinical evidence. Knowledge graphs and representation learning approaches can connect signals across data types to surface targets with stronger mechanistic plausibility and translational relevance. This reduces investment in weak targets and improves the probability that downstream chemistry aligns with meaningful biology.
Virtual screening is another high-leverage domain. Predictive models can rank molecules based on binding likelihood and stability, filter candidates for developability, and triage vast libraries computationally before synthesis. Generative models go further by proposing new chemical matter designed to satisfy multiple constraints at once, including potency, selectivity, solubility, and synthetic accessibility. This shifts medicinal chemistry from broad exploration to guided iteration, increasing the probability that lead series advance with fewer cycles.
ADMET prediction is a third area of compounding value. Many candidates fail due to toxicity, metabolic instability, or poor pharmacokinetics. AI models trained on historical datasets can surface these risks earlier, allowing teams to prioritize molecules with stronger safety and developability profiles before expensive downstream work begins. These predictions do not eliminate the need for experimental validation, but they reduce the probability of late-stage surprise and strengthen the efficiency of early decision-making.
Why Cloud HPC Has Become the Operational Backbone
AI Driven Drug Discovery is not limited by ideas. It is limited by compute, data, and workflow integration. Cloud HPC makes AI Driven Drug Discovery scalable by supporting large-scale training, high-throughput screening, and simulation-driven iteration without fixed infrastructure constraints.
Cloud-native environments also enable modern collaboration patterns that are increasingly essential in discovery. Pharma, biotech, CROs, academia, and automated labs operate as interconnected ecosystems. Cloud platforms support governed data sharing, reproducible pipelines, experiment tracking, and secure multi-party collaboration. When integrated properly, they enable feedback loops where wet-lab results refine models, models inform experiments, and the system improves continuously over time.
This is the foundation of an AI Driven Drug Discovery ecosystem that is not only faster, but also more transparent, auditable, and repeatable across programs and therapeutic areas.
Quantum Computing as a Future Expansion Layer
Quantum computing is not yet a mainstream workhorse in drug discovery, but its potential is increasingly credible as a future layer in the computational stack. The promise lies in simulating molecular behavior with higher fidelity and exploring complex optimization spaces more effectively than classical methods can achieve within realistic time and cost constraints.
Potential applications include improved modeling of binding energies, higher-precision molecular simulation, reaction pathway optimization, and complex network analysis across multi-omics systems. Most near-term value is expected through hybrid approaches where quantum methods are applied to specific sub-problems within classical AI and HPC workflows. Organizations that prepare now through quantum literacy, targeted pilots, and architectural readiness will be better positioned to adopt mature quantum capabilities as they become commercially practical.
How NexRevo Bioinformatics Enables AI Driven Drug Discovery at Scale
AI Driven Drug Discovery delivers value only when it is operationalized through the right architecture, governance, and workflow integration. NexRevo Bioinformatics supports organizations across the full lifecycle by connecting scientific depth with engineering execution.
We help teams establish AI-ready data foundations that unify assay results, omics datasets, chemical libraries, and historical R and D knowledge into governed environments optimized for modeling and traceability. We design cloud-native architectures that support scalable HPC workloads, secure collaboration, and reproducible pipelines. We build and deploy ML workflows for target discovery, virtual screening, ADMET prediction, and candidate prioritization aligned to experimental realities. We also support quantum readiness by identifying high-impact use cases and designing hybrid patterns that reduce future adoption friction.
The outcome is practical acceleration, reduced attrition, and improved predictability across discovery programs.
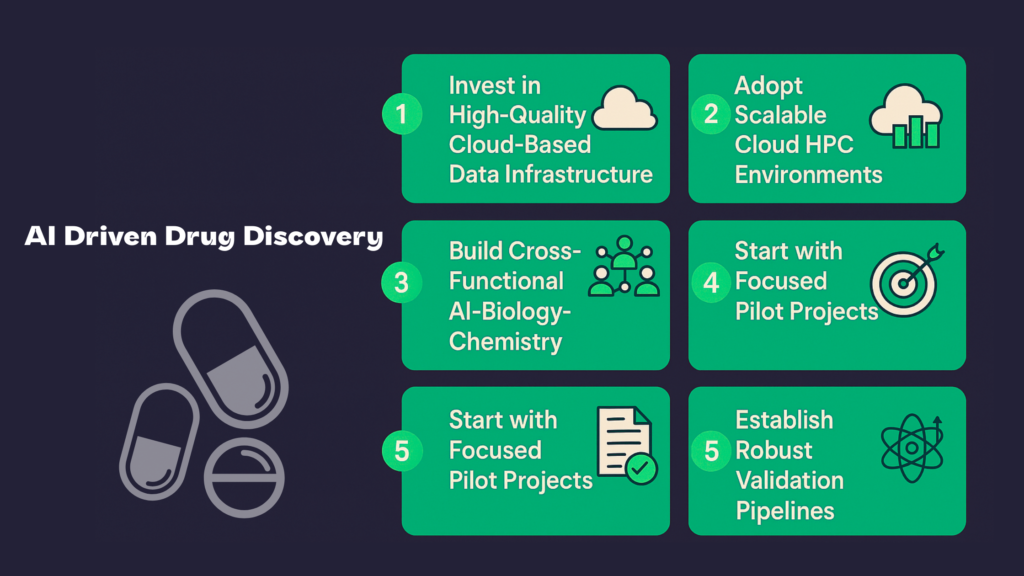
A Practical Path Forward for Discovery Leaders
Organizations that want to capture the value of AI Driven Drug Discovery need more than tools. They need operating foundations that allow AI to be trusted, scaled, and integrated into real decisions.
High-quality data infrastructure is the starting point because model performance is constrained by data completeness, consistency, and governance. Scalable cloud HPC is the execution engine that enables iterative screening, training, and simulation at the pace discovery demands. Cross-functional integration between biology, chemistry, and computation is what ensures predictions translate into experiments that move programs forward. Validation pipelines must be designed into the system so that predictions are tested, refined, and made regulatory-ready over time. Finally, quantum readiness should be approached as strategic preparation, ensuring organizations can adopt new computational capabilities faster as the technology matures.
Conclusion
Drug discovery is shifting from a linear pipeline to a predictive, computationally amplified system. AI Driven Drug Discovery, cloud HPC, and emerging quantum capabilities are redefining how targets are chosen, how molecules are designed, and how risk is surfaced early.
Organizations that invest in data foundations, scalable compute, model governance, and cross-functional workflows will be positioned to lead the next generation of biomedical innovation. NexRevo Bioinformatics is ready to partner with R and D organizations, biotechs, and pharma teams to build intelligent discovery systems and accelerate the journey from insight to therapeutic impact.